PLINABULIN
Plinabulin is a differentiated tubulin binder with potent immunomodulatory and tumor vasculature targeting effects that reinforce the antitumor response of standard of care chemotherapy/radiation and checkpoint inhibition regimens.
Plinabulin is investigational and not FDA approved.
PLINABULIN
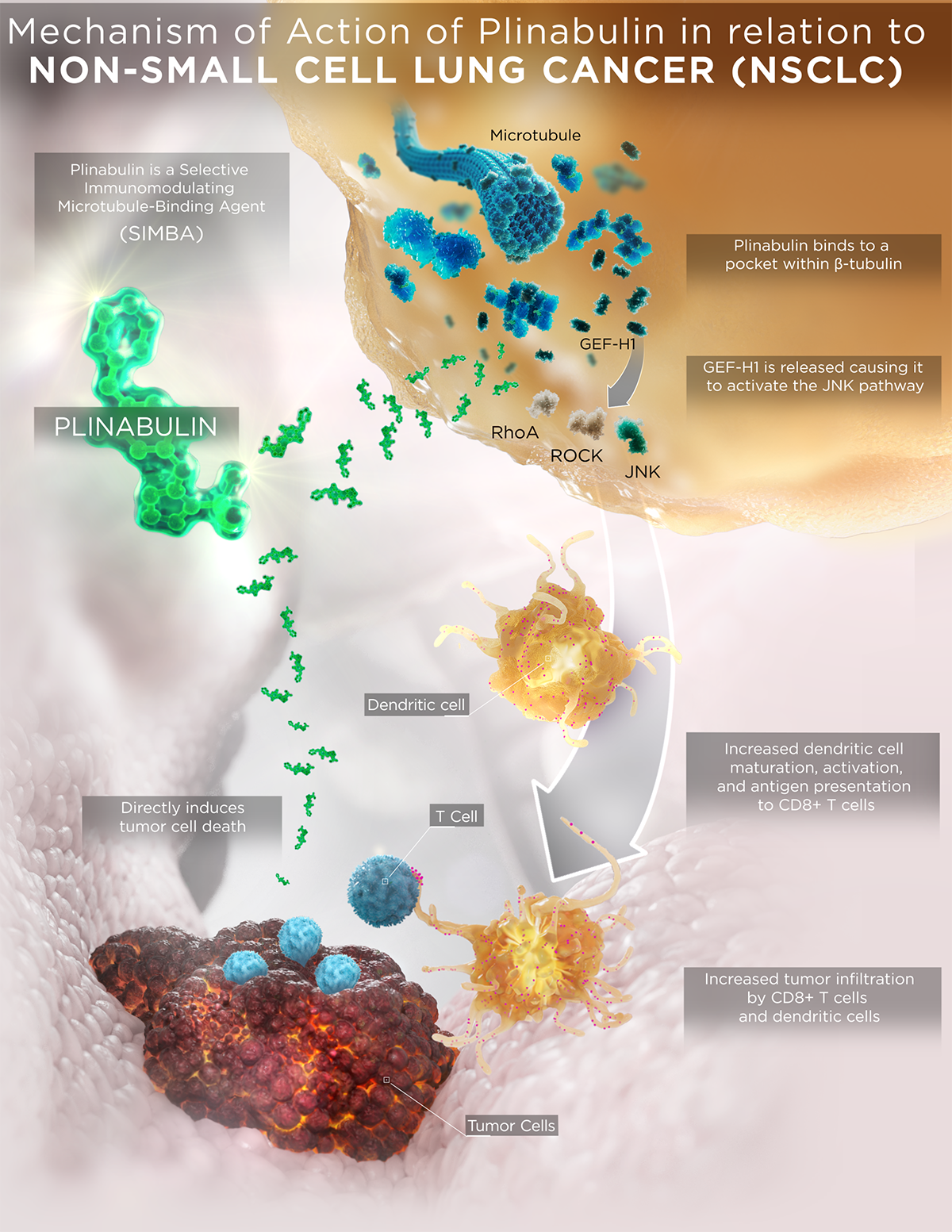
Plinabulin is an investigational immunomodulating small molecule that represents a new class of GEF-H1 activating microtubule-interfering agents. It features a unique chemo-, immuno- and radio-enhancing mechanism of action (MOA) compared to current standard-of-care chemotherapeutics and conventional microtubule-targeting agents, which also result in differentiated safety and efficacy profiles.
Plinabulin binds in a distinct pocket of ß-tubulin with differentiated binding kinetics from other tubulin binding agents, including taxanes, vincas, and colchicine (La Sala 2019 Chem).
- Conventional tubulin binding agents (such as taxanes, vinca alkaloids, and colchicine) disrupt microtubule dynamics. However, plinabulin does not change the apo or unbound structure of tubulin, which results in a reversible and transient interaction with microtubules, with the outcome of releasing immune defense protein GEF-H1 activating dendritic maturation and T cell activation.
- This differentiation in ß-tubulin binding not only results in a well-tolerated safety profile in >700 cancer patients, but also in differentiated immune-modulating biological activities for plinabulin.
PROPOSED MOA
- Plinabulin triggers the release of the immune defense protein, GEF-H1, which leads to a durable anti-cancer benefit due to the maturation of dendritic cells (the most potent antigen resenting cell (APC)), resulting in the activation of tumor antigen-specific T-cells to target cancer cells (Kashyap et al 2019 Cell Reports).
- Plinabulin has antiangiogenic and direct anti-mitotic cancer cell killing activities (Singh et al 2011 Blood).
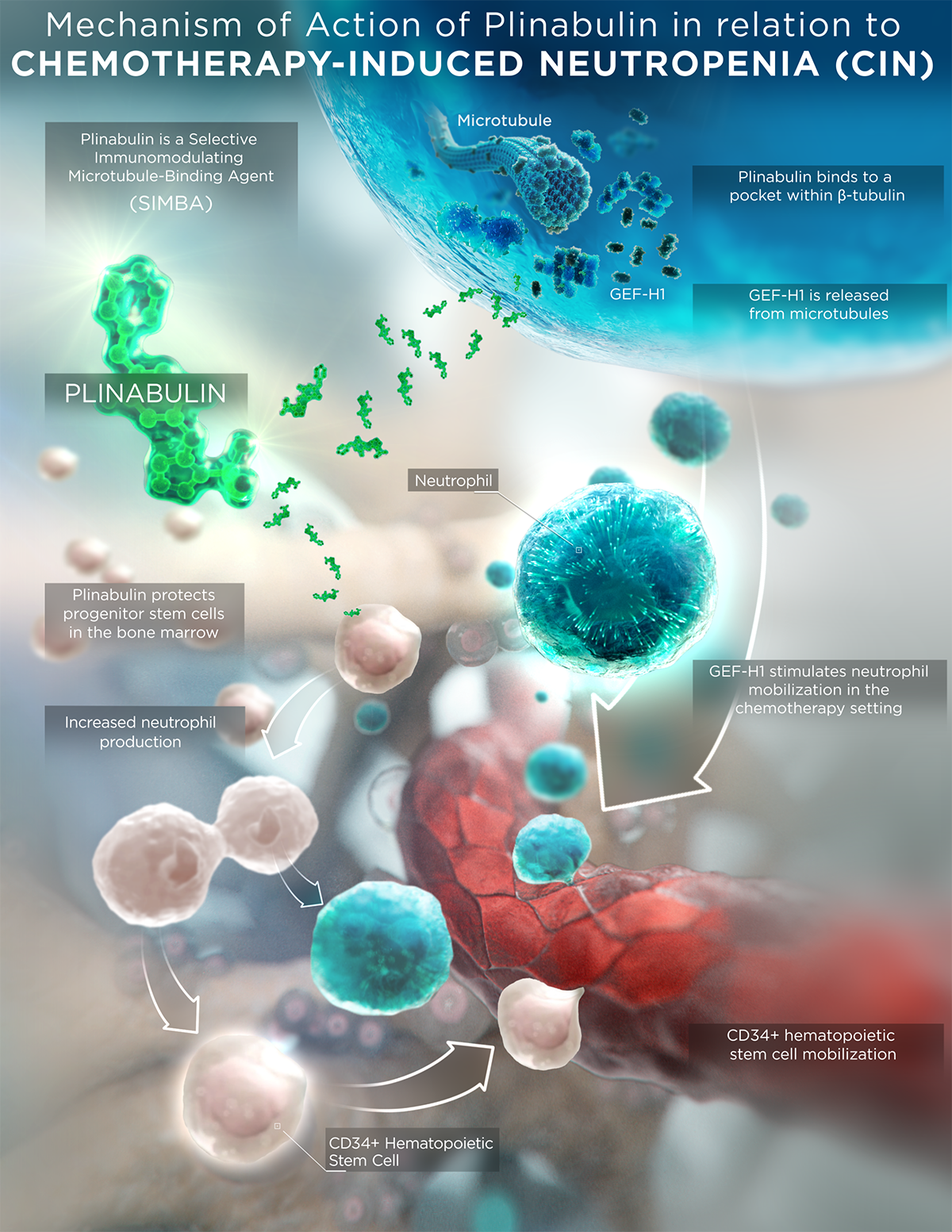
- Plinabulin also exerts an early-onset of action in CIN prevention in week 1 after chemotherapy by boosting the number of hematopoietic stem/progenitor cells (HSPCs) (Tonra et al 2020 Cancer Chemother Pharmacol).
In treating NSCLC
While PD 1/PD-L1 inhibitors have moved into the first line, docetaxel and docetaxel-based regimens remain the standard-of-care in second- and third-line NSCLC without actionable driver mutations. As updated by Mellman et al, a functional cancer-immunity cycle starts when neoantigens are released from dead tumor cells generated by chemotherapy (e.g., docetaxel) and/or radiation. These neoantigens are captured by antigen presenting dendritic cells and presented to T cells in tumor-draining lymph nodes. Upon proper priming and activation, tumor-specific effector T cells infiltrate the tumor microenvironment and ultimately destroy the cancer cells. Thus, harnessing plinabulin’s potent immunomodulatory effect on dendritic cells is a promising strategy to augment the cancer immunity cycle when combined sequentially with chemotherapy or radiation and without or with immune checkpoint inhibitors.
Plinabulin is investigational and not FDA approved.
In preventing CIN
Under myelosuppressive conditions such as chemotherapy-induced neutropenia (CIN), plinabulin displays a unique bone marrow mobilization effect with an anti-CIN mechanism of action distinct from that of granulocyte-colony stimulating factor (G-CSF), with differentiated safety profile of limited bone pain.
Plinabulin is investigational and not FDA approved.
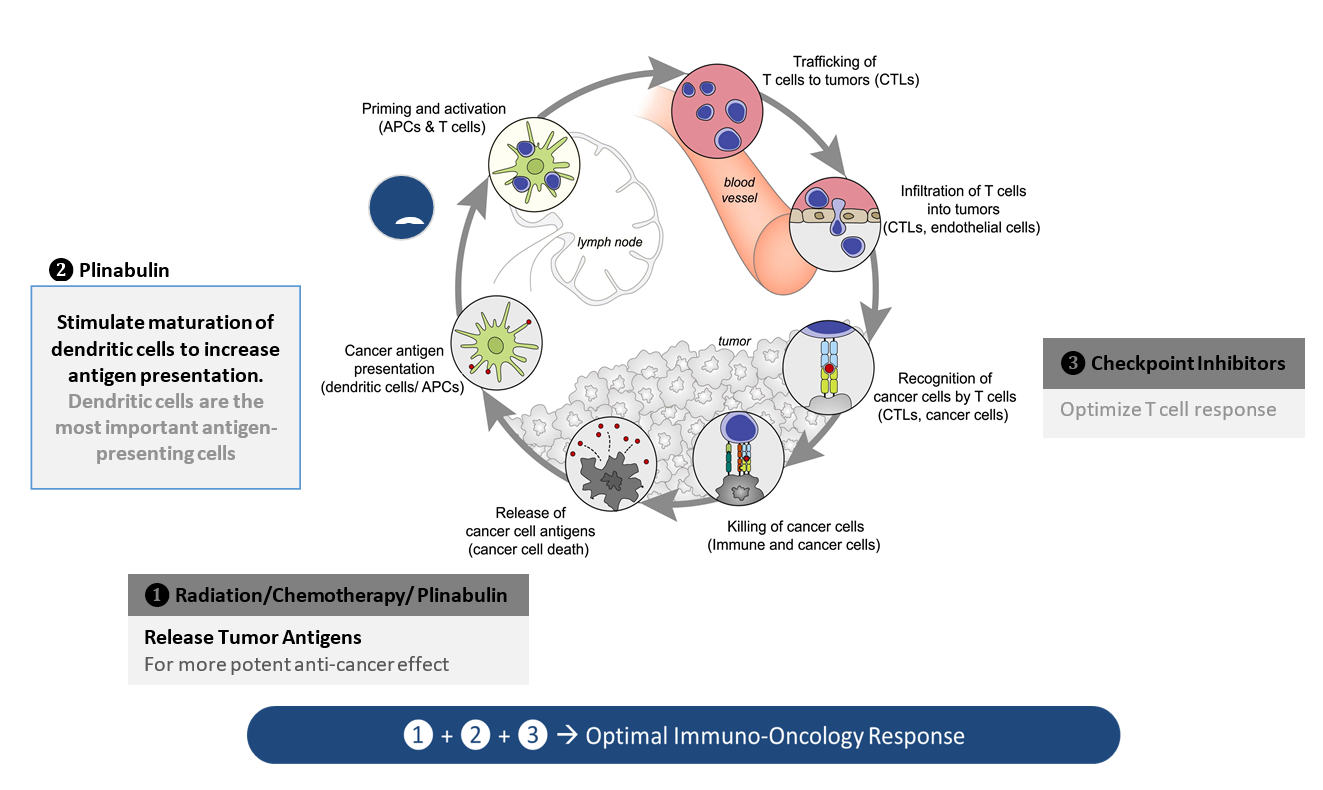
Adapted from: Mellman, D.S. Chen, T. Powles, S.J. Turley, The cancer-immunity cycle: Indication, genotype, and immunotype, Immunity, 56 (2023) 2188-2205. The cancer-immunity cycle: Indication, genotype, and immunotype: Immunity
With these unique MOAs, Plinabulin is being developed as a “pipeline in a drug”, in a number of cancer indications as a direct anti-cancer agent for indications with severe unmet medical needs. Plinabulin had shown a balanced profile with encouraging durable anti-cancer benefit and prevention of chemotherapy-induced neutropenia.
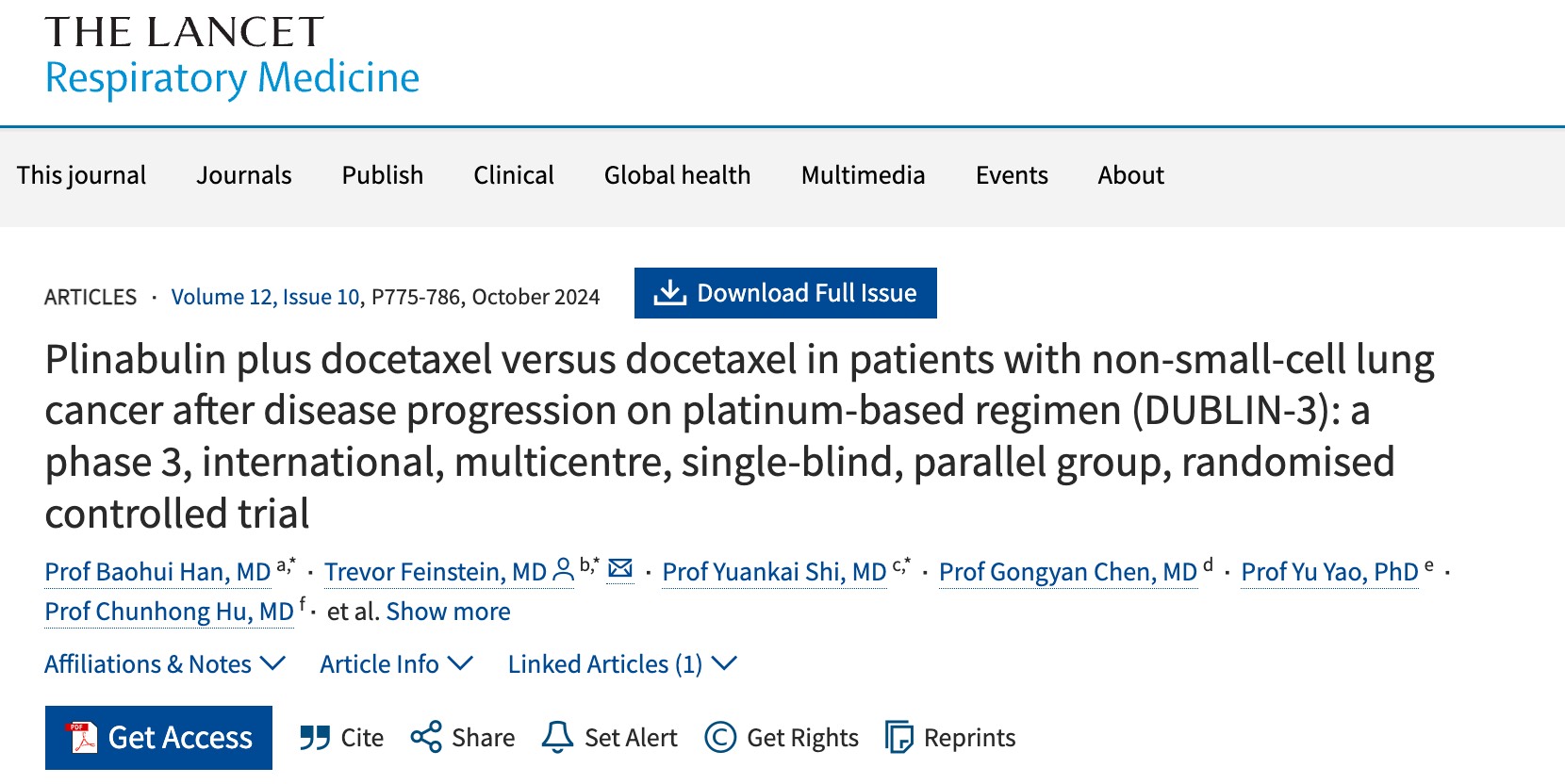
- In the Phase 3 DUBLIN-3 study (n=559), the plinabulin and docetaxel combination met the primary endpoint of extending overall and long-term survival compared to docetaxel alone in second- or third-line NSCLC, EGFR wild type (Shi et al 2024 Lancet RM).
- In Phase 1/2 IIT Big Ten Study, plinabulin combined with PD-1 and CTLA-4 antibodies had promising safety data in second- or third-line ES-SCLC who failed platinum and PD-1/PD-L1 antibodies (Malhotra et al 2024 Lung Cancer).
- In Phase 2 IIT metastatic NSCLC with disease progression after prior PD-1/PD-L1 inhibitors, plinabulin in combination with docetaxel and PD-1 inhibitor had 89.3% disease control rate and median PFS of 8.6 months in 30 patients (2024 SITC poster).
- In Phase 1 IIT study at MD Anderson, plinabulin in combination with radiotherapy and PD-1 inhibitors in a number of cancer types with disease progression after prior immunotherapy (2023 SITC poster).
- In Phase 2 IIT study in first line ES-SCLC, plinabulin in combination with etoposide/platinum and PD-1 inhibitor (NCT05745350), as well as
- In phase 2 and 3 studies, plinabulin as a monotherapy or in combination with G-CSF, showed clinically meaningful results in prevention of chemotherapy-induced neutropenia (2021a Blayney ASCO; 2021b Blayney ASCO; 2021c Blayney ASCO).
MOA video animation
Plinabulin is investigational and not FDA approved.
The highly differentiated MOA is published in numerous industry journals, including Chem and Cell Reports. Click a publication below to read the full article.
CHEM
Structure, Thermodynamics, and Kinetics of Plinabulin Binding to Two Tubulin Isotypes

CELL REPORTS
GEF-H1 Signaling upon Microtubule Destabilization is Required for Dendritic Cell Activation and Specific Anti-tumor Responses

NSCLC
Second- and third-line NSCLC with EGFR wild type patients are patients with severely unmet medical needs. While PD‑1/PD-L1 inhibitors have moved to first line, docetaxel and docetaxel-based regimens remain the standard of care in second and third line, with an approximately 10% 2‑year survival rate, and around a 5% 3‑year survival rate and >40% severe neutropenia.
In the last 10 years, no new agent has been approved in this indication.
CLINICAL TRIALS
For details of this trial, please visit below website.
TRIPLE IO COMBINATIONS
PLINABULIN + DOCETAXEL + PEMBROLIZUMAB (303 Study)
This is an open-label, single-arm phase 2 IIT study at Peking Union Medical College Hospital (China) testing plinabulin in combination with docetaxel and pembrolizumab in previously treated patients with metastatic NSCLC and progressive disease after anti-PD-(L)1 antibody alone or in combination with platinum-doublet chemotherapy. All patients (47 planned) would have experienced disease progression after initial clinical benefit with ICI prior to the trial entry. On day 1 of each 21-day cycle, plinabulin (30mg/m2 IV) was intentionally administered 1 hour after docetaxel (75 mg/m2 IV) followed by pembrolizumab (200 mg IV).
At the database lock on 29 August 2024, 36 patients were enrolled, 30 exposed to the plinabulin regimen. At a median follow-up time of 11.5 months, the disease control rate including partial responses and stable disease (> 4 months) was 89.3% with median PFS of 8.6 months compared to median PFS of 5.5 months in a similar patient setting but treated with “pembrolizumab + docetaxel” [26]. The most common adverse events were myelosuppression, GI side effect, and transient hypertension with no treatment-related deaths (SITC 2024 poster).
CLINICAL TRIALS
For details of this trial, please visit website below.
PLINABULIN + ETOPOSIDE/PLATINUM + PEMBROLIZUMAB (302 Study)
This is an open-label, single-arm, phase 2 IIT Study at Wuhan Union Hospital (China) testing plinabulin in combination with etoposide/platinum and pembrolizumab as 1L therapy for extensive-stage (ES)-SCLC. All patients (45 planned) enrolled are receiving the following interventional treatments. On day 1 of each 21-day cycle, plinabulin (30mg/m2 IV) was intentionally administered 1 hour after etoposide (100 mg/m2 IV)/carboplatin (AUC 5 IV) or cisplatin (75 mg/m2 IV) followed by pembrolizumab (200 mg IV). Other than safety evaluations, the primary endpoint is 12-month PFS rate with secondary ORR, duration of response, PFS and OS endpoints.
CLINICAL TRIALS
For details of this trial, please visit website below.
PLINABULIN + PD-1/PD-L1 + RADIATION
This is an open-label, single-arm phase 1/2 IIT study at MD Anderson Cancer center testing plinabulin in combination with radiation and anti-PD-1 antibody in selected cancer types with progressive disease after prior PD-(L)1 therapy. All patients (with at least two lesions, 1 irradiated and 1 unirradiated) at the study initiation received the triple combination treatment of RT + plinabulin + ICI in C1, followed by continued ICI and plinabulin combination regimen until disease progression, unacceptable toxicity, patient withdrawal, or treatment discontinuation per study design. A short course of local consolidative RT (8 Gy x 3, 12.5 Gy x 4, or 4 Gy x 5) was administered in C1, starting from D1. Plinabulin (30 mg/m2 IV) was dosed 3–6 hours later on D1 and D4 of C1 in combination with an ICI at the dose and schedule according to the FDA recommendation. Pembrolizumab (200 mg IV) was given on D1 of every treatment cycle (21 days) or nivolumab (240 mg IV) once every 14 days. The primary endpoints were safety, tolerability, and ORR along with secondary endpoint (disease control rate, DCR) and exploratory biomarkers focusing on DC maturation and GEF-H1 immune activation.
At the data cutoff on February 15, 2024, a total of 19 patients out of the 29 screened received at least 1 dose of plinabulin combined with RT in 3 or 4 fractions plus ICI (14 on pembrolizumab and 5 on nivolumab). This triple regimen was safe and achieved a DCR of 54% (3/13 PR and 4/13 SD > 6 months). Responding tumors included NSCLC (2/2 PR+SD), head-and-neck-squamous-cell-carcinoma (1/3 PR, 1/3 SD and 1/3 clinical progressive disease) and Hodgkin’s lymphoma (2/2 PRs in patients after 12- or 16-prior lines-of-therapy). Importantly, PR+SD patients had significantly higher GEF-H1 immune activation scores in peripheral blood and intra-tumorally at pre-treatment/baseline and DC activation/T-cell small clonal expansion post-treatment. These preliminary results provide a rationale for testing RT/plinabulin/ICI combination in post-ICI-failure confirmatory trials. In addition, we found higher tumor proportion and higher ARG1 expression in monocyte-derived macrophages in PD patients, which likely contributed to an immune-resistant phenotype of the tumor microenvironment (SITC 2023 poster).
CLINICAL TRIALS
For details of this trial, please visit website below.
PLINABULIN + NIVOLUMAB + IPILIMUMAB (Big Ten Study)
This was an investigator-initiated phase 1/2 clinical trial with a triple combination therapy, consisting of plinabulin, nivolumab, and ipilimumab, for the treatment of 2nd and 3rd line small cell lung cancer. The trial, conducted through the Big Ten Cancer Research Consortium, which includes Rutgers Cancer Institute of New Jersey and other clinical centers in the U.S. This study investigated whether the addition of plinabulin results in a reduction of immune-related side effects of PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies and if it provides efficacy synergy.
Plinabulin in combination with nivolumab and ipilimumab was tolerable at the dose of 30 mg/m2. While the clinical responses in PD-1 resistant SCLC were limited, some patients had a long duration of response. The number of ≥grade 3 irAE with the combination was lower than expected. The result of this study was published. (Malhotra et al 2024 Lung Cancer)
CLINICAL TRIALS
For details of this trial, please visit website below.
Presentation of Chemotherapy-induced Neutropenia (CIN)
Plinabulin is being evaluated for the prevention of CIN and is in two global multi-center clinical trials aiming for a broad indication of prevention of all CIN in all non-myeloid cancer types.
PROTECTIVE-1 (Study 105) is a phase 2/3 trial of plinabulin versus Neulasta, a long lasting granulocyte-colony stimulating factor (G-CSF), after a standard regimen of docetaxel, for advanced breast cancer, hormone-refractory prostate cancer and advanced NSCLC patients (Blayney et al 2020 JAMA Oncology, Blayney et al 2022 JAMA Network).
PROTECTIVE-2 (Study 106) is a phase 2/3 trial of plinabulin in combination with Neulasta (plinabulin/pegfilgrastim combination) versus plinabulin monotherapy versus Neulasta monotherapy after a high-risk myelosuppressive chemotherapeutic regimen composed of three agents, Taxotere (docetaxel), Adriamycin (doxorubicin) and Cytoxan (cyclophosphamide), or TAC, for patients with advanced breast cancer (2021b Blayney ASCO; 2021c Blayney ASCO).
These clinical studies showed a differentiated clinical profile of Plinabulin in the prevention of CIN from G-CSF:
- Early onset of action in Week 1 in preventing CIN,
- Limited bone pain, and
- Limited immune-suppression.
CLINICAL TRIALS
For details of these two trials, please visit below websites.