
PIPELINE OVERVIEW
We have a robust drug development pipeline at various clinical and preclinical stages.
Tap the sections below to expand or download the full pipeline chart.
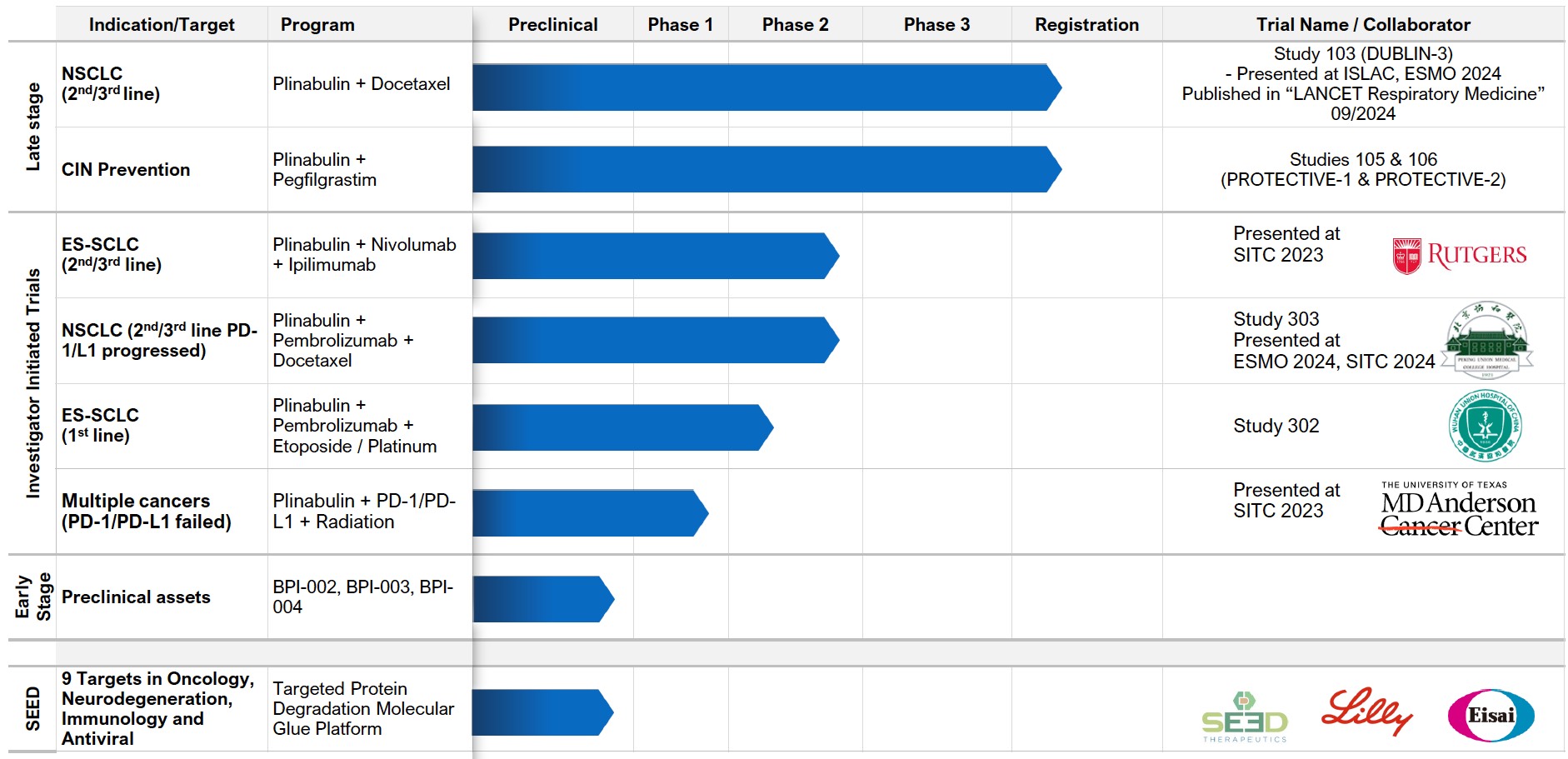

We have a robust drug development pipeline at various clinical and preclinical stages.
Tap the sections below to expand or download the full pipeline chart.
