Plinabulin is an investigational small molecule that is in the new SIMBA class
Plinabulin, a selective immunomodulating microtubule-binding agent (SIMBA), has a mechanism of action (MOA) that is different from current standard-of-care chemotherapeutics and conventional microtubule-binding agents. This unique MOA has led to the investigation of plinabulin for the indications of:
- The prevention of chemotherapy-induced neutropenia (CIN) in combination with G-CSF
- The treatment of late-stage non-small cell lung cancer (NSCLC) and other solid tumors
MOA video animation
Plinabulin is investigational and not FDA approved.

How plinabulin works
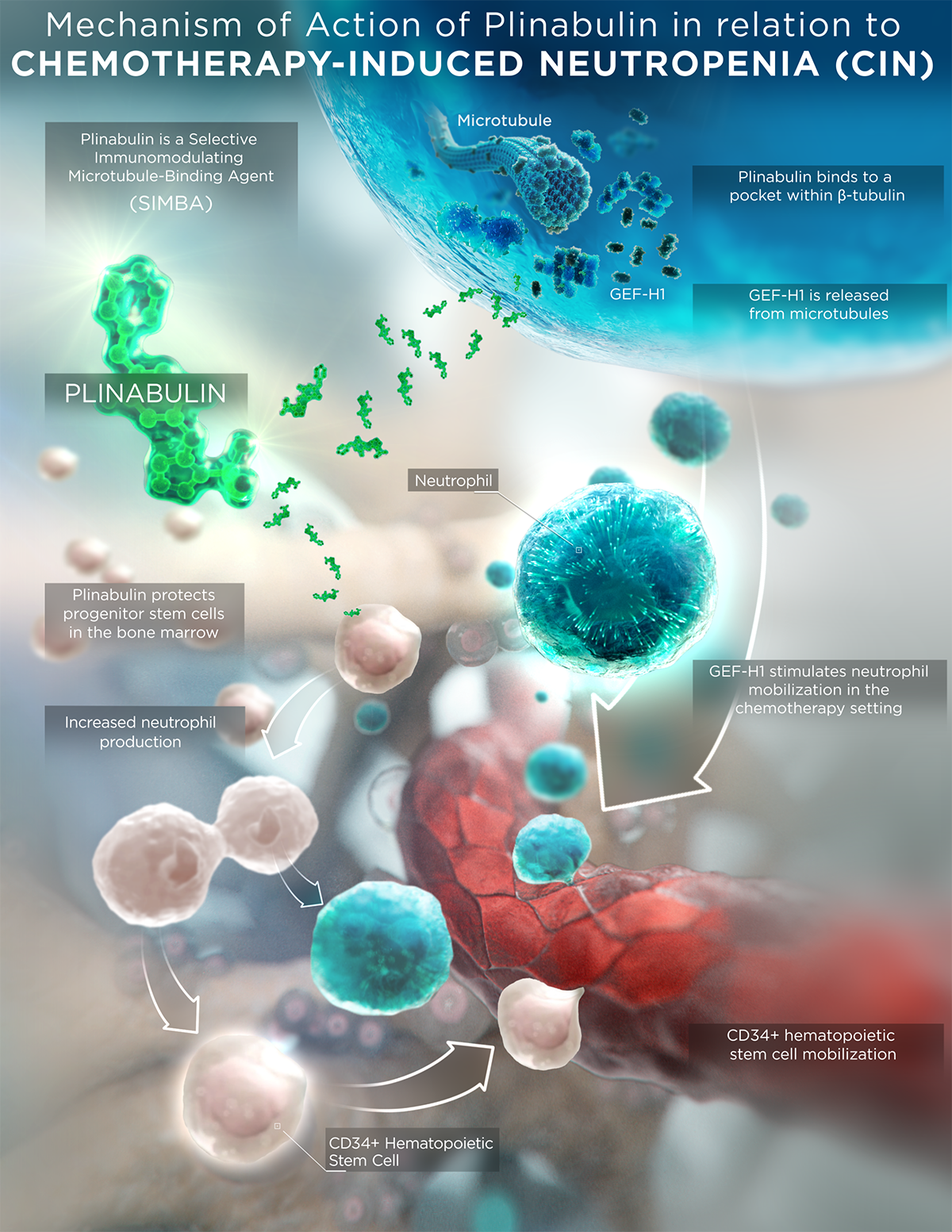
As a SIMBA, plinabulin binds to microtubules at a unique site on tubulin – leading to the release of GEF-H1
from microtubules.2-7
Plinabulin’s MOA in detail
Plinabulin’s immunomodulatory MOA drives increased neutrophil production by hematopoietic stem cells in the bone marrow.9
Studies have shown that through GEF-H1 activation, plinabulin plays a role in protecting progenitor hematopoietic stem cells in bone marrow. Plinabulin mobilizes CD34+ hematopoietic stem cells, helping reduce their numbers in bone marrow.10
In addition, plinabulin maintained median absolute neutrophil counts within normal range, whereas patients given G-CSF experienced median absolute neutrophil counts higher than the normal range, which can potentially cause bone marrow exhaustion.11
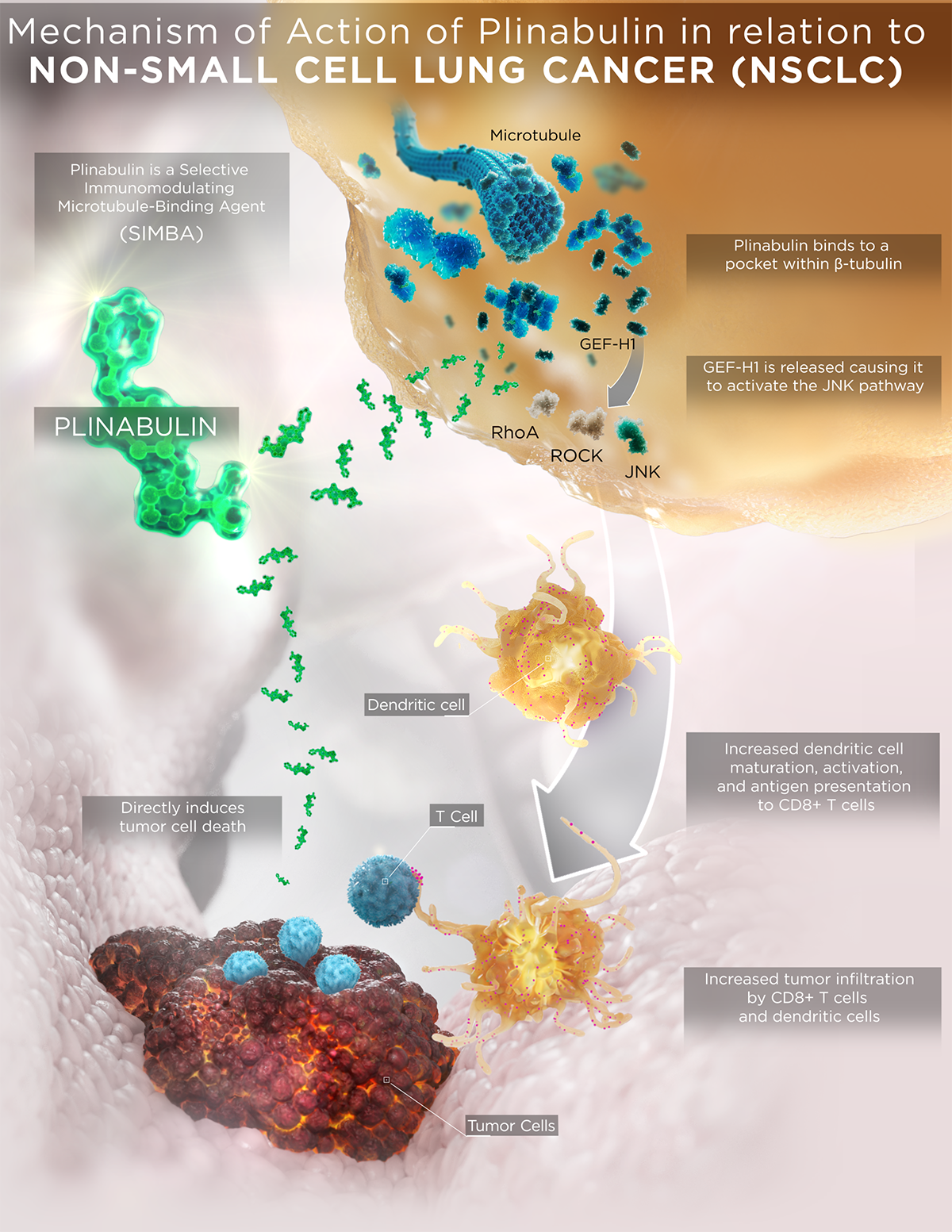
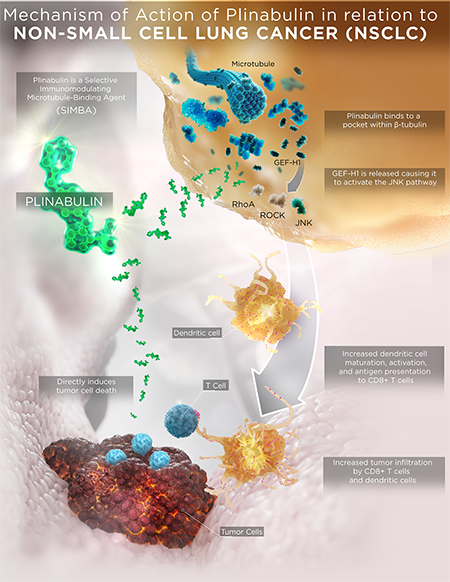
Plinabulin modulates pathways that have an indirect and direct effect on cancer cells
Plinabulin’s SIMBA properties enhance maturation of dendritic cells, which stimulates T cells, leading to death of cancer cells. This immunomodulatory action on the immuno-oncology system also enhances tumor infiltration. Addition of plinabulin to a radiotherapy + anti-PD1 immunotherapy regimen potentially increases tumor infiltration by CD8+ T cells and dendritic cells.12-14
Plinabulin modulates pathways that have an indirect and direct effect on cancer cells
Plinabulin’s SIMBA properties enhance maturation of dendritic cells, which stimulates T cells, leading to death of cancer cells. This immunomodulatory action on the immuno-oncology system also enhances tumor infiltration. Addition of plinabulin to a radiotherapy + anti-PD1 immunotherapy regimen potentially increases tumor infiltration by CD8+ T cells and dendritic cells.12-14
SIMBA Posters
From the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting
ASCO Poster 1
A phase I trial of plinabulin in combination with nivolumab and ipilimumab in patients with relapsed small-cell lung cancer (SCLC): Big Ten Cancer Research Consortium (BTCRC-LUN17-127) study
ASCO Poster 2
Chemotherapy Induced Profound Neutropenia (PN) in Patients (pts) with Breast Cancer (BC) after chemotherapy and Plinabulin (Plin) plus Pegfilgrastim (Peg) Combination versus (vs) Peg Alone. Final Phase 3 Results from PROTECTIVE-2 (BPI-2358-106)
ASCO Poster 3
Clinical Trial Testing Superiority of Combination Plinabulin and Pegfilgrastim vs Pegfilgrastim Alone in Patients with Breast Cancer Treated with High Febrile Neutropenia Risk Chemotherapy: Final Results of the Phase 3 Chemotherapy-Induced Neutropenia Prevention Trial (PROTECTIVE-2)
REFERENCES
- Wang Y, Probin V, Zhou D. Cancer therapy-induced residual bone marrow injury- mechanisms of induction and implication for therapy. Curr Cancer Ther Rev. 2006;2(3):271-279.
- Nicholson B, Lloyd GK, Miller BR, et al. NPI-2358 is a tubulin-depolymerizing agent: in-vitro evidence for activity as a tumor vascular-disrupting agent. Anticancer Drugs. 2006;17(1):25-31.
- Wang Y, Zhang H, Gigant B, et al. Structures of a diverse set of colchicine binding site inhibitors in complex with tubulin provide a rationale for drug discovery. FEBS J. 2016;283(1):102-111.
- Heck JN, Ponik SM, Garcia-Mendoza MG, et al. Microtubules regulate GEF-H1 in response to extracellular matrix stiffness. Mol Biol Cell. 2012;23(13):2583-2592.
- Fine N, Dimitriou ID, Rullo J, et al. GEF-H1 is necessary for neutrophil shear stress-induced migration during inflammation. J Cell Biol. 2016;215(1):107-119.
- Data on file. BeyondSpring Inc.; 2016.
- Hettinger K, Vikhanskaya F, Poh MK, et al. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 2007;14(2):218-229.
- Blayney DW, Ogenstad S, Ette E, et al. Plinabulin-associated neutrophil demargination: evidence for a clinically relevant mechanism of action for the prevention of chemotherapy-induced neutropenia. Presented at: Society for Leukocyte Biology Meeting; October 13-16, 2018; Chandler, AZ.
- Ghosh A, Zhu Y, Fan Z, et al. Plinabulin ameliorates chemotherapy-induced neutropenia: mechanistic insights. Presented at: American Association for Cancer Research (AACR) Annual Meeting; April 14-18, 2018; Chicago, IL.
- Blayney DW, Ogenstad S, Ette E, et al. Plinabulin, a Novel Small Molecule in Development for Chemotherapy-Induced-Neutropenia (CIN) Prevention, Mobilizes CD34+ Cells through a Mechanism of Action Different from G-CSF and from CXCR4 Inhibition. Presented at: American Society of Hematology Meeting; December 1-4, 2018; San Diego, CA.
- Data on file. BeyondSpring Inc.; 2020.
- Kashyap AS, Fernandez-Rodriguez L, Zhao Y, et al. GEF-H1 signaling upon microtubule destabilization is required for dendritic cell activation and specific anti-tumor responses. Cell Rep. 2019;28(13):3367-3380.e8.
- Neri S, Sharma A, Lloyd GK, et al. Plinabulin, a microtubule destabilizing agent, improves tumor control by enhancing dendritic cell maturation and CD8 T cell infiltration in combination with immunoradiotherapy. Abstract 4449. Presented at: AACR Annual Meeting; April 27-28, 2020 and June 22-24, 2020; Philadelphia, PA.
- Mohanlal RW, Blayney D, Lloyd K, et al. Plinabulin, a Novel Small Molecule Clinical Stage IO Agent with Anti-Cancer Activity, Prevents Chemo-Induced Neutropenia, and has the Potential to also Prevent Immune Related AEs. Presented at: ASCO-SITC Clinical Immuno-Oncology Symposium; January 25-27, 2018; San Francisco, CA.